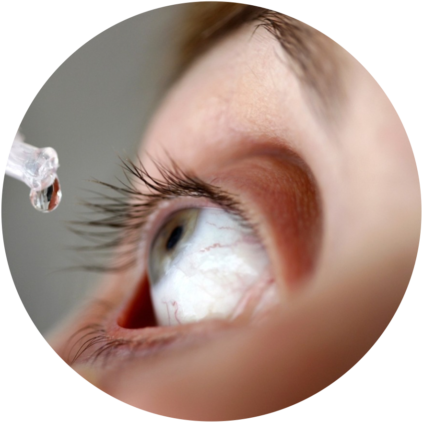
A clear lack of innovation in ophthalmic drug delivery Eye diseases are currently addressed only via 2 ways

Jean GARREC, Pharm D Founder & CEO
Jean holds a Pharm D. and an MBA with 20 years business experience in the field of ophthalmic industry. He gained an excellent understanding of the market, challenges, and players in eye pharmaceuticals. Jean held several general management experiences for building and steering companies, that provided him the required expertise in global business development, market access, and government price negotiations.

Jean CUINÉ, Pharm D, PhD Co-founder and CTO
Jean holds a Pharm.D., a Ph.D. in Pharmaceutical Sciences and an MBA. He has 20 years international experience and a track record developing new drugs from ideation to market. Jean started his career as a Research Scientist in drug delivery at Monash University prior to lead global development organizations and programs portfolio at executive level at Stallergenes Greer, Nextpharma, Cenexi and Novartis.

Alexandre BOULAY, PhD GO Capital
Alexandre holds a PhD in Chemistry with a degree in chemisty applied to Life Sciences and a master in Corporate Finance. After several years in consulting then as a Technology Transfer Officer, he joined GO Capital in 2020 where he is currently Senior Associate serving on the board of several companies in Healthtech and Deeptech.

Valérie CALENDA, PhD Mérieux Equity Partners
Valérie holds a PhD in Pharmacology with a degree in Hematology and a B.Sc. in Biochemistry-Immunology. She is a former research scientist in Hematology and Virology at INSERM and then transitioned to Transgene (affiliate of Institut Mérieux) focusing on gene therapy and immunotherapy. Valérie joined Mérieux Equity Partners in January 2010 as Managing Partner, serving on the board of companies in Europe, Israel and North America.

Florian DENIS, MsC Elaia partners
Florian holds a MsC from Master MTI (Management of Technology and Innovation) – Université Paris Dauphine – Mines Paristech – ENS and Sup’Biotech Paris. He joined Elaia in 2019 and is an active member of the life sciences investments. He started his career in venture capital in 2015 at Auriga Partners. Florian serves on the Board of Directors of several companies and is responsible for the exit of Mablink Bioscience which was sold to Eli Lilly.

Valentin T. FLEURY, MsC HTL Biotechnology
Valentin obtained a MsC from Ecole Centrale Lyon. He was strategy consultant for healthcare and life sciences companies. As an advisor and board member, he has supported the development of numerous start-ups, notably in ophthalmology. During his various tenures he engaged several strategic alliances and M&A. He currently serves as Head of Strategy at HTL Biotechnology, where he adeptly conceived and led HTL’s diversification strategy.

Nathalie MASSON, Pharm D Unither pharmaceuticals
Nathalie holds a Pharm D. and an MSc in Process Engineering. She gained more than 30 years experience in pharmaceutical development at the global level for Rx pharmaceuticals, OTC, cosmetics and food supplements. Nathalie currently serves as the Director of Innovation and Development at Unither pharmaceuticals.
Our investors
Our partners
-
- Statista – Kantar Media 2021 UK study in 2020 on 24,024 respondents aged 15 years and older
- WHO publication, 2019 World Report on Vision
- Behar-Cohen et al. – SFO report 2015
- Global Data report: Glaucoma Global Drug Forecast and Market Analysis to 2026
- Okeke et al. – Ophthalmology Feb. 2009