Eye disease management still suffers from important limitations:
For front of the eye diseases, progressive & irreversible vision losses are often caused by high rates of non-compliance to daily eyedrop treatments : 45% glaucoma patients1 don’t respect their prescriptions (<80% of the dose)2 leading to reduced efficacy.
Only 5% of the drug content in an eyedrop will be absorbed by the eye, meaning that 95% is wasted in tears and blinking.
Since poor yield eyedrops are not suitable to treat back of the eye diseases, retinal disorders require invasive intra-ocular injections. Performed at the hospital by an ophthalmologist, these injections generate stress & pain for the patients & high associated costs for the healthcare systems (treatment, hospital fees).
Most patients regularly forget about their eye drops, favoring their disease progression.
Too frequent injections cause patients to stop their treatment.
Eye disease management still suffers from important limitations:
For front of the eye diseases, progressive & irreversible vision losses are often caused by high rates of non-compliance to daily eyedrop treatments : 45% glaucoma patients1 don’t respect their prescriptions (<80% of the dose)2 leading to reduced efficacy.
Only 5% of the drug content in an eyedrop will be absorbed by the eye, meaning that 95% is wasted in tears and blinking.
Since poor yield eyedrops are not suitable to treat back of the eye diseases, retinal disorders require invasive intra-ocular injections. Performed at the hospital by an ophthalmologist, these injections generate stress & pain for the patients & high associated costs for the healthcare systems (treatment, hospital fees).
Most patients regularly forget about their eye drops, favoring their disease progression.
Too frequent injections cause patients to stop their treatment.
Eye disease management still suffers from important limitations:
For front of the eye diseases, progressive & irreversible vision losses are often caused by high rates of non-compliance to daily eyedrop treatments : 45% glaucoma patients1 don’t respect their prescriptions (<80% of the dose)2 leading to reduced efficacy.
Only 5% of the drug content in an eyedrop will be absorbed by the eye, meaning that 95% is wasted in tears and blinking.
Since poor yield eyedrops are not suitable to treat back of the eye diseases, retinal disorders require invasive intra-ocular injections. Performed at the hospital by an ophthalmologist, these injections generate stress & pain for the patients & high associated costs for the healthcare systems (treatment, hospital fees).
Most patients regularly forget about their eye drops, favoring their disease progression.
Too frequent injections cause patients to stop their treatment.
BIOPHTA’s mini-tablet (3mm diameter) is simply placed on the surface of the eye as easily as a contact lens.
Once applied, BIOPHTA’s ophthalmic insert transforms into a hydrogel pellet that stays in place on the eye without moving, and delivers a continuous and controlled low dose of drug for 7 days. It’s the first time a therapy has such innovative technological features to disrupt both front-of-the-eye as well as retina treatments.
BIOPHTA’s mini-tablet (3mm diameter) is simply placed on the surface of the eye as easily as a contact lens.
Once applied, BIOPHTA’s ophthalmic insert transforms into a hydrogel pellet that stays in place on the eye without moving, and delivers a continuous and controlled low dose of drug for 7 days. It’s the first time a therapy has such innovative technological features to disrupt both front-of-the-eye as well as retina treatments.
BIOPHTA has developed a new standard of care for ocular diseases based on its “thiomer” biopolymers technological platform, with a strong intellectual property position (3 patent families).
This technology offers four unique dedicated features:
Initially in dry & solid form to facilitate its self-application by the patients, our treatment instantly transforms into a hydrogel when it comes into contact with the tearfilm of the eye and avoids causing any foreign body sensation.
Creating covalent bonds with the natural mucins of the eye surface allows our hydrogel to perfectly stay in place for 7 days.
Biopolymer matrix composed of a tight nanofiber network enables the slow release of the drug content in a fine and continuous microdosing.
Topical treatment of the retina, thanks to an increase of the ocular drug absorption by the specific properties of these novel biopolymers.
Initially in dry & solid form to facilitate its self-application by the patients, our treatment instantly transforms into a hydrogel when it comes into contact with the tearfilm of the eye and avoids causing any foreign body sensation.
Creating covalent bonds with the natural mucins of the eye surface allows our hydrogel to perfectly stay in place for 7 days.
Biopolymer matrix composed of a tight nanofiber network enables the slow release of the drug content in a fine and continuous microdosing.
Topical treatment of the retina, thanks to an increase of the ocular drug absorption by the specific properties of these novel biopolymers.
BIOPHTA overcomes adherence issues encountered with traditional eye drops as well as the high cost and invasiveness of frequent and painful intraocular injections. With this unique topical, non-invasive and self-administered therapy that provides continuous drug microdosing for 7 days, BIOPHTA improves the efficacy of eye treatments.
It’s the first time a therapy has such innovative technological features to disrupt both front-of-the-eye as well as retina treatments.
Pipeline
Our non-invasive and topical ophthalmic Inserts have the ability to be used with multiple therapeutic compounds to target several indications.
We plan ultimately to use our unique ophthalmic insert technology to address retinal diseases, begining with macular edema ($9.6 billion global market value) in replacement of current intra-ocular injections.
Hence, our roadmap is primarily driven by the rapid initiation of a First in Human pilot study that can be enabled in 2025 thanks to a formulation of our sustained release insert in the treatment of glaucoma. Approximately 76 million people worldwide are affected by glaucoma which accounts for a $8.7 billion global prescription eyedrops market. This First in Human pilot study using our ophthalmic insert will then allow us to develop other formulations for initiating additional programs in parallel.
Our unique ophthalmic insert technology has the potential to be used across multiple indications
Major Eye diseases to be treated by BIOPHTA’s game-changing platform
Eye Surface

ALLERGIC CONJUNCTIVITIS 120 Million patients
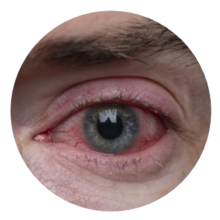
DRY EYE SYNDROME 150 Million patients
Anterior Segment

GLAUCOMA 76 Million patients
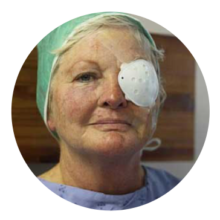
POST-SURGICAL TREATMENT 28 Million cataract surgeries / year
Retina

DIABETIC RETINOPATHY 146 Million patients
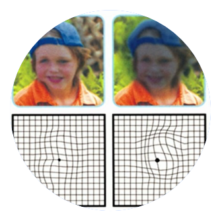
AGE-RELATED MACULAR DEGENERATION 196 Million patients
Our unique ophthalmic insert technology has the potential to be used across multiple indications
Major Eye diseases to be treated by BIOPHTA’s game-changing platform
Eye Surface

ALLERGIC CONJUNCTIVITIS 120 Million patients

DRY EYE SYNDROME 150 Million patients
Anterior Segment

GLAUCOMA 76 Million patients

POST-SURGICAL TREATMENT 28 Million cataract surgeries / year
Retina

DIABETIC RETINOPATHY 146 Million patients

AGE-RELATED MACULAR DEGENERATION 196 Million patients
Our unique ophthalmic insert technology has the potential to be used across multiple indications
Major Eye diseases to be treated by BIOPHTA’s game-changing platform
Eye Surface

ALLERGIC CONJUNCTIVITIS 120 Million patients

DRY EYE SYNDROME 150 Million patients
Anterior Segment

GLAUCOMA 76 Million patients

POST-SURGICAL TREATMENT 28 Million cataract surgeries / year
Retina

DIABETIC RETINOPATHY 146 Million patients

AGE-RELATED MACULAR DEGENERATION 196 Million patients
-
- Okeke et al. – Ophthalmology, Feb. 2009
- Newman-Casey et al. – Ophthalmology, Oct. 2015
